How Many Valence Electrons Does Astatine Have
Valence electrons in Neon Ne 8. Valence electrons in Sodium Na 1.

Astatine At Element 85 Of Periodic Table Elements Fllashcards
The sulfur atom in SF 4 has 10 valence electrons and 12 valence electrons in SF 6.

. The nucleus is composed of protons and neutrons. There are 32 valence electrons available for the Lewis structure for SO 4 2. Therefore the valence electrons of argonAr are eight.
Valence electrons in Nitrogen N 5. We have shown the Valence Electrons of the elements for which reliable data is available. Atomic Symbol At State at 20 C solid Description Unstable radioactive member of the halogen group.
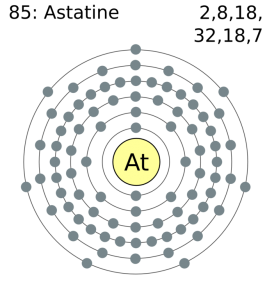
The last shell after the electron configuration is called the valence shell. In an electrically neutral atom of astatine there are 85 electrons. The ability of one atom of an element to join another atom during the formation of a molecule is called valencyvalence.
How many protons does 135 Ba2 have. Best Answer Copy Astatine has a valency of 1 because it is in group 7 and is part of the halogens. Valence electrons in Fluorine F 7.
The main group number for an element can be found from its column on the periodic table. For example carbon is in group 4 and has 4 valence electrons. How many valence electrons does s have in so42.
85 At - Astatine. Likewise what is the condensed electron configuration of zirconium. How many electrons are in Na.
The number of protons is equal to the atomic number the big number by the atom on a periodic table The number of electrons is equal to the number of protons The number of neutrons is equal to the atomic mass minus the atomic number Denise May 25 2016. There are only four valence electrons for this answer. The total number of electrons in a valence shell is called a valence electron.
How Many Valence Electrons Does Astatine Have. Since the last shellorbit of a gallium ion has eighteen electrons the valence electrons of gallium ionsGa 3 are eighteen. May 25 2016 The element Astatine has 85 protons as well as 85 electrons.
Valence electrons in Oxygen O 6. Astatine has 39 isotopes and they are all radioisotopes. 86 Rn - Radon.
There are some rules for diagnosing valency. Atoms of Group 17 the halogens have 7 valence electrons. Since astatine is a halogen it follows the same pattern as the other halogens and has seven valence electrons.
The chemical symbol for Astatine is At. They say the group number is the number of valence but the valency cant be 7. How to determine the valency of gallium.
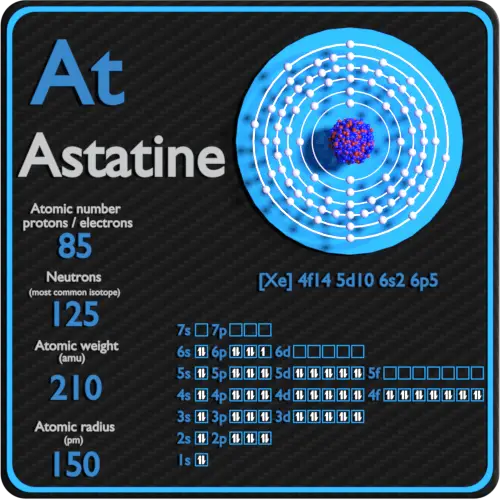
Group 15 has 5 valence electrons. The electronic configuration of Astatine is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2 6p 5. These elements include fluorine chlorine bromine iodine and astatine.
Metalloids can either lose or share electrons when they combine with other elements. 22 Pauling scale Crystal structure of Astatine predicted FCC face centered cubic Density of Astatine. Valence electrons in Astatine.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. What charge does astatine have. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus.
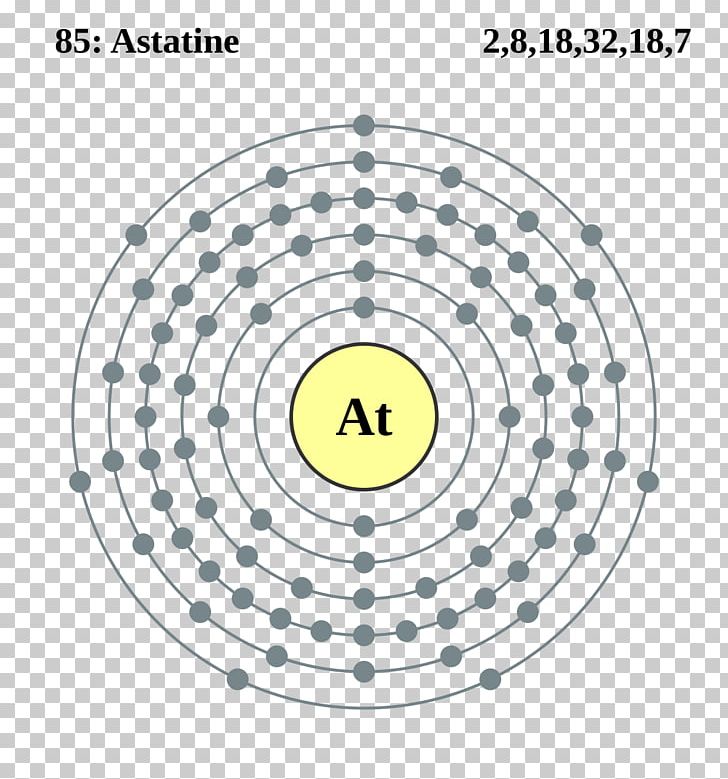
Ba2 ion has 56 protons and 54 electrons. Hence the astatine element has electrons arrangement 2 8 18 32 18 7. It will still have 11 positive protons but only 10 negative electrons.
How many valence electrons does group 15 have. Accordingly how many valence electrons are in an atom. Each hydrogen atom has one valence electron and is univalentThe number of valence electrons.
Valence electrons in Aluminum Al 3. Is valency and valence electrons the same. Valence electrons in Carbon C 4.
So they do 8 the. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom. Likewise people ask how many valence electrons does zirconium have.
A valence electron makes six electrons of oxygen and holds 7 electrons of lead. Now lets check the facts about Astatine. Any element in the halogen group will have seven valence electrons.
Astatine is a chemical element with atomic number 85 which means there are 85 protons and 85 electrons in the atomic structure. Its oxidation states are 1 1 3 5 7 and its electro negativity is 22 Pauling scale. Therefore the number of electrons in neutral atom of Astatine is 85.
Why does group 17 have 7 valence electrons. A gain of one or more electrons gives these atoms a stable 8 electrons so the halogens react easily with other elements. 119 rows Valence electrons in Boron B 3.
A Silicon molecule consists of five electrons 2 8 electrons 4 5 115 21 20 21 21 24 31 or 32 electrons. Valence Electrons Graph - Valence Electrons of all the elements in graph. Like iodine astatine has been shown to adopt odd-numbered oxidation states ranging from 1 to 7.
Uses Since its isotopes have such short. Astatine Overview Astatine Valence Electrons 1357 Atomic Number 85 Learn more about the atomic number. So the overall charge is 1.
87 Fr - Francium. Zirconium atoms have 40 electrons and the shell structure is 28. It has a very unstable nature and is also very rare in.
Any element in the halogen group will have seven valence electrons. The electron configuration of argon shows that the last shell of argon has eight3s 2 3p 6 electrons. Atomic Mass 2099871 Learn more about the atomic mass.
Any element in the halogen group will have seven valence electrons. 1st Ionization energy of Astatine. Which group has all 7 valence electrons.
Valence electrons in Magnesium Mg 2. These elements include fluorine chlorine bromine iodine and astatine. How Many Valence Does Silicon Have.
These elements include fluorine chlorine bromine iodine and astatine. How many valence electrons does the noble family have. For neutral atoms the number of valence electrons is equal to the atoms main group number.
How can you determine the number of valence electrons in an atom. For neutral atoms the number of valence electrons is equal to the atoms main group number. Valency is the number of bonds an element or an atom can form.
Eight valence electrons Click to see full answer.

Astatine Atomic Structure Stock Image C018 3766 Science Photo Library

Periodic Table Element Comparison Compare Iodine Vs Astatine Compare Properties Structure Facts

Astatine Atomic Structure Stock Image C045 6432 Science Photo Library
How To Find The Electron Configuration For Astatine At
Astatine Electron Shell Chemical Element Radon Radium Png Clipart Angle Area Astatine Atom Atomic Number Free
File Astatine Shells Svg Wikimedia Commons
Superhero Astatine By Miyaaa Jackson

Density Of The Ilmos Of The Astatine Anion Surrounded By Ten Water Molecules The Last Three Framed Orbitals Are Non Relativistic Spinors Thus The Possibility To Show Phases

Astatine Facts Symbol Discovery Properties Uses

Periodic Table And Valence Electron Quiz In 2022 Periodic Table Physical Science Electrons

Comparing Halogen Reactivity Trends With Those Of The Alkali Metals Halogens Become Less Reactive Down The Chemistry Classroom High School Chemistry Chemistry
Astatine Protons Neutrons Electrons Electron Configuration
How Many Valence Electrons Does Astatine Have Quora
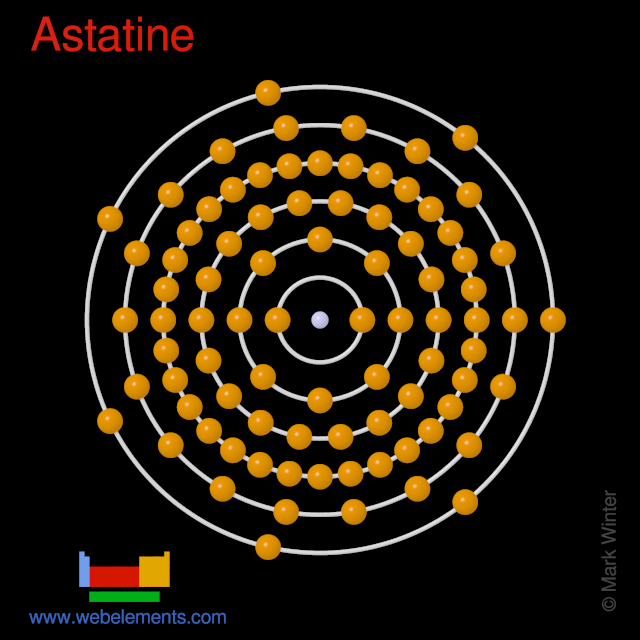
Webelements Periodic Table Astatine Properties Of Free Atoms

Pin By Abdulrahman Alghamdi On Chemistry Made Easy Homeschool Kindergarten Middle School Science Experiments Infographic

2022 Valence Electrons In Astatine At Facts Color Discovery

Astatine Facts Symbol Discovery Properties Uses

Chemical Elements Color By Symbols 3 Element Symbols Science Lessons Classroom Activities

Comments
Post a Comment